ALZHEIMER’S DISEASE STUDY HIGHLIGHTS – Clinical Meaningfulness in Alzheimer’s Disease Clinical Trials. A Report from the EU-US CTAD Task Force by Angioni, D., Cummings, J., Lansdall, C.J. et al.
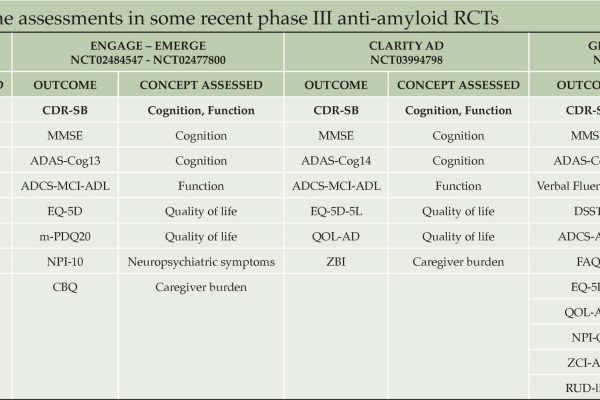
In their study, Angioni et al. report several general principles identified by the EU-US CTAD Task Force that should be considered when evaluating the meaningfulness of clinical trial results. The most commonly used primary outcomes in Alzheimer’s clinical trials, such as the Clinical Dementia Rating Scale sum of boxes (CDR-SB) and the integrated Alzheimer’s Disease Rating Scale (iADRS), capture concepts that are meaningful to patients and care partners while also reflecting the core manifestations of disease. Secondary and exploratory outcomes support the observed effects on cognition and function, as well as the impact of treatment on other important concepts for patients and carers (e.g., neuropsychiatric symptoms, carer burden, and quality of life). Meaningful within-person change thresholds are not intended to specify the magnitude of between-group differences in mean change from baseline. Further research, possibly in real-world settings, is required to evaluate the generalisability of existing thresholds for meaningful within-person change on CDR-SB and iADRS.
Access the full article at: Angioni, D., Cummings, J., Lansdall, C.J. et al. “Clinical Meaningfulness in Alzheimer’s Disease Clinical Trials. A Report from the EU-US CTAD Task Force”. J Prev Alzheimers Dis (2024). https://link.springer.com/article/10.14283/jpad.2024.112. This article is licensed under a CC-BY license.