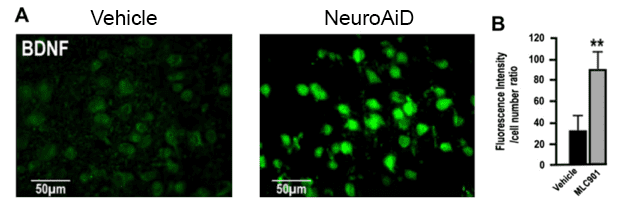
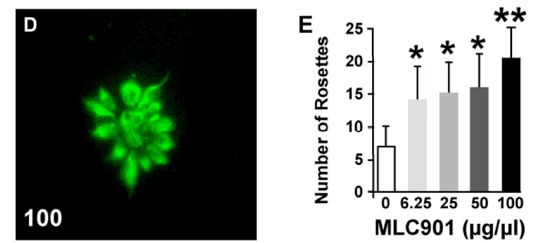
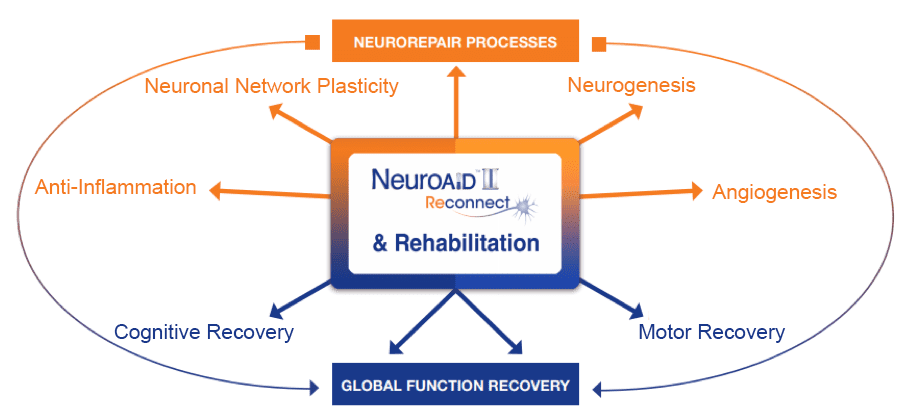
Amplification of brain self-repair processes translating into persistent functional recovery.
A 3-month course with NeuroAiD:
-
Increases by 50% the odds of achieving functional independence at 6 months.1
-
Enhances persistent functional recovery and independence on top of rehabilitation for up to 2 years.1,2,3
-
Improves motor recovery with benefits observed as early as 1 month of treatment and at each time point up to 3 months.4
The CHIMES-E Study, functional recovery over 2 years.1
-
Pre-planned study on 880 subjects* with Acute Ischaemic Stroke followed up for 2 years.
-
Randomised to 3 months of either NeuroAiD or placebo.

+50% in odds of achieving functional independence at 6 months.
Persistent recovery with benefits observed over 2 years
In subjects with 2 to 4 prognostic factors of poorer outcome** and receiving NeuroAiD2 :
-
More subjects achieved functional independence over time versus placebo.
-
Significant improvements observed as early as 3 months.
-
Less subjects deteriorating over time versus placebo, illustrating the persistence of the recovery
*CHIMES-E published in
Cerebrovascular Diseases journal is the pre-planned extended follow-up to 24 months of the CHIMES study
5, a 3-month study conducted on 1099 subjects. The CHIMES study was published in
Stroke journal in 2013.
**Prognostic factors are defined as older age, female sex, baseline NIHSS score ≥ 10 and time to first dose 48h.
Combining NeuroAiD and Rehabilitation doubles the odds of achieving functional independence versus Rehabilitation alone.3
-
Analysis of 380 subjects having received rehabilitation during 3 months of NeuroAiD (n=200) or placebo (n=180).

+50% in odds of achieving functional independence at 6 months.
Persistent recovery with benefits observed over 2 years
-
Statistically significant odds ratio at all time points from 3 months to 2 years from stroke onset.
-
Persistent recovery with benefits sustained for up to 2 years, as supported by low Numbers Needed to Treat (NNT) range from 5 to 7 over the 24-month follow-up period.
NeuroAiD improves motor recovery.4
-
Randomised double-blind study placebo-controlled study in post-acute stroke subjects with upper and lower limbs deficits, included up to one month following an ischaemic stroke (n=150).

Significantly better motor recovery observed since month 1 and sustained up to month 3.
References:
- Venketasubramanian N, et al. CHInese Medicine NeuroAiD Efficacy on Stroke recovery – Extension Study (CHIMES-E): A multicenter study of long-term efficacy. Cerebrovascular Dis. 2015; 39:309-318.
- Venketasubramanian N, et al. Prognostic Factors and Pattern of Long-Term Recovery with MLC601 (NeuroAiD™) in the Chinese Medicine NeuroAiD Efficacy on Stroke Recovery – Extension Study. Cerebrovascular Dis. 2017; 43:36–42.
- Suwanwela NC, et al. Effect of Combined Treatment with MLC601 (NeuroAiDTM) and Rehabilitation on Post-Stroke Recovery: The CHIMES and CHIMES-E Studies. Cerebrovasc Dis. 2018 doi: 10.1159/000492625.
- Harandi AA, et al. Safety and efficacy of MLC601 in Iranian patients after stroke: a double-blind, placebo-controlled clinical trial. Stroke Research and Treatment. 2011; doi:10.4061/2011/721613.
- Chen C, et al. Chinese medicine Neuroaid efficacy on stroke recovery – A double-blind, placebo-controlled, randomized study. Stroke. 2013; 44:2093-2100.
- Theadom A, et al. MLC901 (NeuroAid II™) for cognition after traumatic brain injury: A pilot randomised clinical trial. Eur J Neurol. 2018;25(8):1055-e82.
- Pakdaman H, et al. MLC901 for moderate to severe traumatic brain injury: pilot, randomised, double-masked, placebo-controlled trial. Open Acc J Comp & Alt Med. 2020; 2(5).OAJCAM.MS.ID.000148. DOI: 10.32474/OAJCAM.2020.02.000148.